FIRST ON FOX — The Food and Drug Administration is phasing out an animal testing requirement for antibody therapies in favor of testing drugs on “lab-grown human ‘organoids,’” the FDA announced on Thursday.
“For too long, drug manufacturers have performed additional animal testing of drugs that have data in broad human use internationally. This initiative marks a paradigm shift in drug evaluation and holds promise to accelerate cures and meaningful treatments for Americans while reducing animal use,” FDA Commissioner Martin A. Makary, said in comment provided to Fox News Digital.
“By leveraging AI-based computational modeling, human organ model-based lab testing, and real-world human data, we can get safer treatments to patients faster and more reliably, while also reducing R&D costs and drug prices. It is a win-win for public health and ethics.”
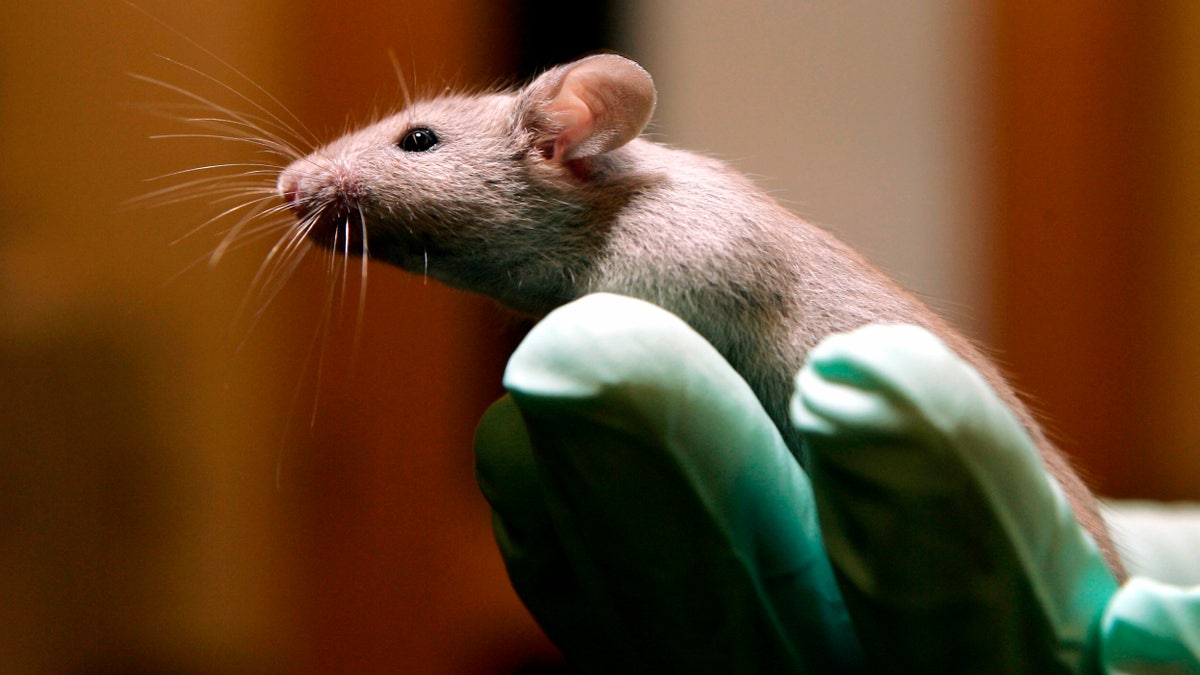
A technician holds a laboratory mouse at the Jackson Laboratory, Jan. 24, 2006, in Bar Harbor, Maine. The lab ships more than two million mice a year to qualified researchers. Eight years ago, a team of researchers launched a project to carefully repeat influential lab experiments in cancer research. They recreated 50 experiments, the type of work with mice and test tubes that sets the stage for new cancer drugs. They reported the results Tuesday, Dec. 7, 2021: About half the scientific claims didn’t hold up. (AP Photo/Robert F. Bukaty, File)
The phase-out focuses on ending animal testing in regard to researching monoclonal antibody therapies, which are proteins made in a lab that are meant to stimulate your immune system to fight diseases such as cancer.

Dr. Martin Makary, President Donald Trump’s nominee to lead the Food and Drug Administration testifies during his confirmation hearing before the Senate Committee on Health, Education, Labor, and Pensions Committee at the Dirksen Senate Office Building on March 06, 2025 in Washington, DC. Dr. Makary is a cancer surgeon, researcher at Johns Hopkins University, and has also been a Fox News commentator. (Kayla Bartkowski/Getty Images)
Instead, according to the FDA’s press release obtained by Fox Digital, the FDA will test on “organoids,” which are artificially grown masses of cells.
“The FDA will promote the use of lab-grown human ‘organoids’ and organ-on-a-chip systems that mimic human organs – such as liver, heart, and immune organs – to test drug safety. These experiments can reveal toxic effects that could easily go undetected in animals, providing a more direct window into human responses,” the press release says.